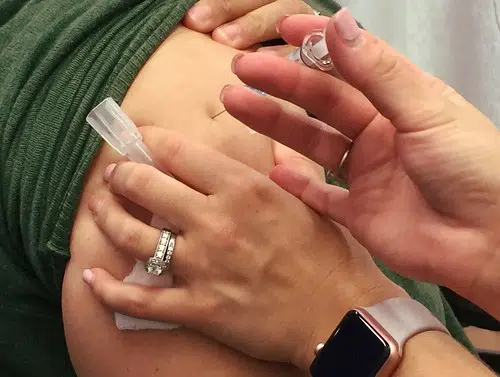
A vaccine for COVID-19 could be ready by the early part of 2021.
Johnson & Johnson is one of more than 100 companies at various phases of clinical trials.
It’s now entering a third phase with their potential vaccine being tested on people.
Infectious Disease Specialist Dr. Dushyantha Jayaweera says their trial will take two years, but they may be ready for market in the new year.
I think March or April we may have some information. It’s hard to say, but the other vaccines are ahead of us, for example, Pfizer, Moderna. They started two months ahead of us,” says Dr. Jayaweera.
More than 60,000 people from around the world will be involved in Johnson & Johnson’s trials.
It’s placebo-controlled. It’s randomized, so we don’t know who gets what. Then we look for the fine points and side effects,” says Dr. Jayaweera.
Johnson & Johnson is one of six companies now into phase three.
President of the American Medical Association, Dr. Susan Bailey, calls the work unprecedented.
“There’s an incredible amount of activity that’s going on in this area which I just think it’s absolutely amazing,” says Dr. Bailey.
Canada has deals with five companies, including Johnson & Johnson, for millions of doses of the vaccine when it becomes available.
Health Canada still has to sign off before it can be used.